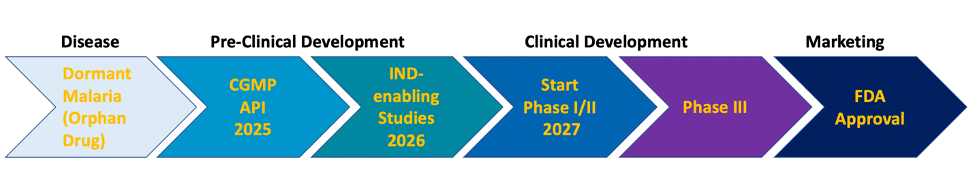
 The broad spectrum activity of Cethromycin affords development of this antibiotic for multiple indications, dramatically increasing the odds of bringing Cethromycin to market. Our initial focus will be on dormant liver-stage malaria.
The broad spectrum activity of Cethromycin affords development of this antibiotic for multiple indications, dramatically increasing the odds of bringing Cethromycin to market. Our initial focus will be on dormant liver-stage malaria.
Cethromycin would be eligible for Fast Track and Breakthrough Therapy designation, Priority Review and Tropical Disease Priority Review Voucher (PRV). Additionally, Cethromycin may qualify for various development incentives of the Orphan Drug Act, including tax credits. Last but not least, Cethromycin is active against Qualified Infectious Diseases pathogens, adding 5 years of marketing exclusivity.